Forecasting Medical Supplies
The Process of Forecasting Medical Supplies requires the coordination and communication between different actors. In this wiki you can find information on how this process evolves from start to end. Overall the process is logically separated into five different sub-processes (actions) that are explained below.
Contents
Call for forecast
Forecasting is the process of calculating the needs of a country with respect to medical supplies for a given NTD, for a certain period of time. The calculation of the quantity needed for a country is performed by the country officials (i.e., Ministries of Health) in collaboration with WHO medical officers at the respective countries (i.e., WHO Country Office and WHO Regional Office). Once the quantity is calculated, the medical supplies need to be requested from WHO. This calculation is performed on a periodic fashion (e.g., yearly), and the actor that actually initiates the process is NTD Programme Manager at WHO HQ. Thus, every year, at a given period of time the Programme Manager (WHO-HQ) sends automatic emails through WIMEDS to notify countries that they can start their Forecasting process. The way this is triggered in WIMEDS is shown visually in the Call for forecasting figure.
Call for forecast that requires a set of approvals
In certain scenarios the emails that are sent by the NTD Programme Manager can be received by Ministries of Health, only if they have first been approved by the WHO Regional Office and the WHO Country Office. WIMEDS is configurable in order to support such cases. This is depicted in Call for Forecast with and without approvals.
Perform forecast
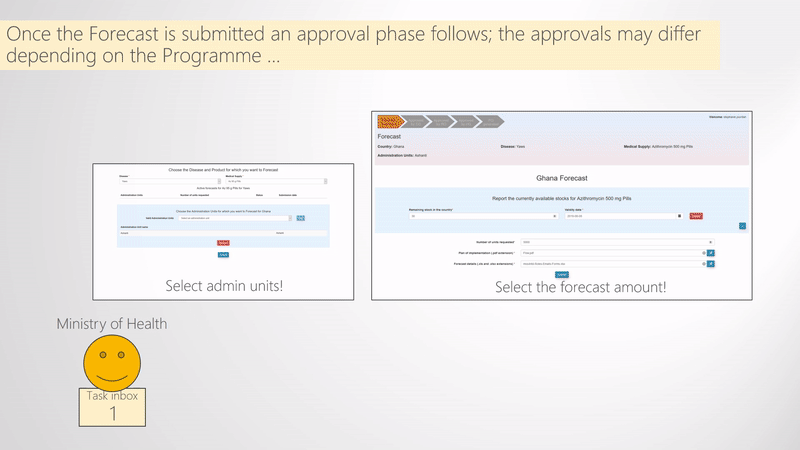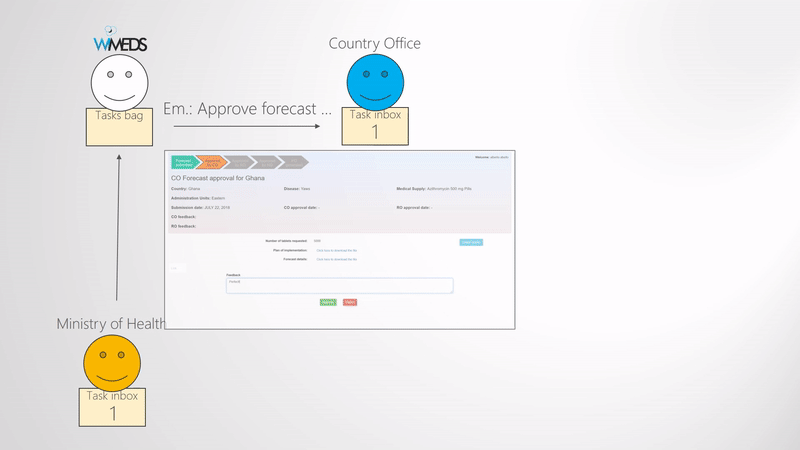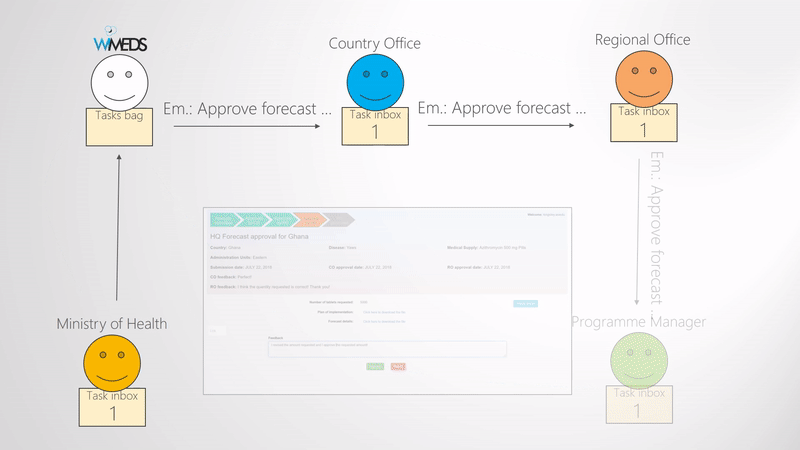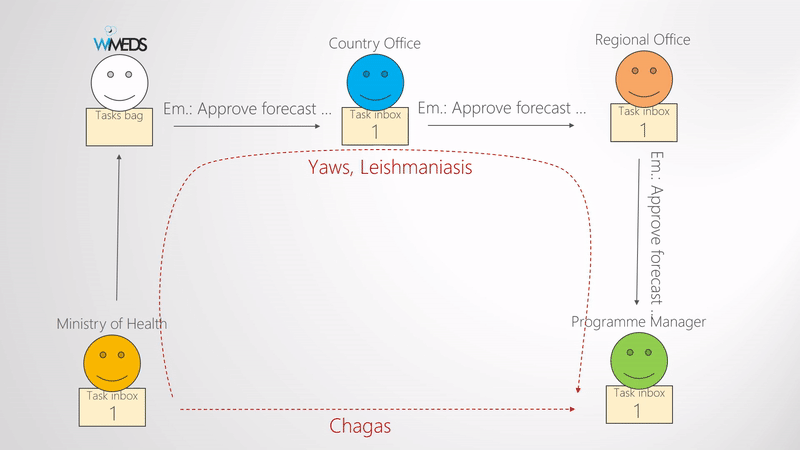
When Ministries of Health receive an email that notifies them that they can perform their forecast, they need to access a web-application that allows them to do so. The process of submitting the Forecast consists of first selecting the Disease, Medical supply, and the Administrative divisions for which the countries want to forecast. Next, in some cases countries may need to report the Available stocks, select the Quantity of the required medical supply and upload the Documents that provide details on the forecasted numbers. A visual representation of how this is done in WIMEDS is shown in the Submit forecast figure.
Select disease, medical supply, and administrative divisions
WIMEDS can be used for forecasting medical supplies for different NTD programmes. Therefore, the first thing that needs to be selected is the Programme/Disease for which the country wants to submit a forecast (typically the Country needs to sign an agreement with the NTD Programme first). Next, since NTD programmes may distribute different medical supplies like medicines and diagnostic kits, the ministry of health needs to first select the Medical supply. Finally, the last thing to be selected in this phase are the Administrative divisions (e.g., regions, cities, etc.) for which the country is forecasting.
Report stocks, insert the quantity of forecast, and upload necessary documents
After the first form is filled as explained above, the ministry of health needs to fill the information about the Stocks with respect to the medical supply selected in the previous phase. Next, the information to be provided is the Forecasted quantity and the necessary Documents that define the Plan of implementation (a pdf file) and the Forecast details (an Excel document). Whne this information is submitted, the forecast process starts and that is explained next.
Approve forecast
When the Ministry of Health submits the forecast, a forecast approval phase is started. What needs to be approved and validated is the quanity of medical supplies and the corresponding documents. The approval phase typically consists of three steps, namely, approval from the WHO Country Office, approval from the WHO Regional Office, and the NTD Programme Manager (i.e., WHO Headquarters). Since some of the programmes may not need all the three levels of approval, this part is also configurable. The way this is done in WIMEDS is visually shown in the Forecast approval phases figure. Note the blue dashed arrows indicate the notification emails sent during the forecast approval phase. That is, all the actors are notified during the different steps of approval.
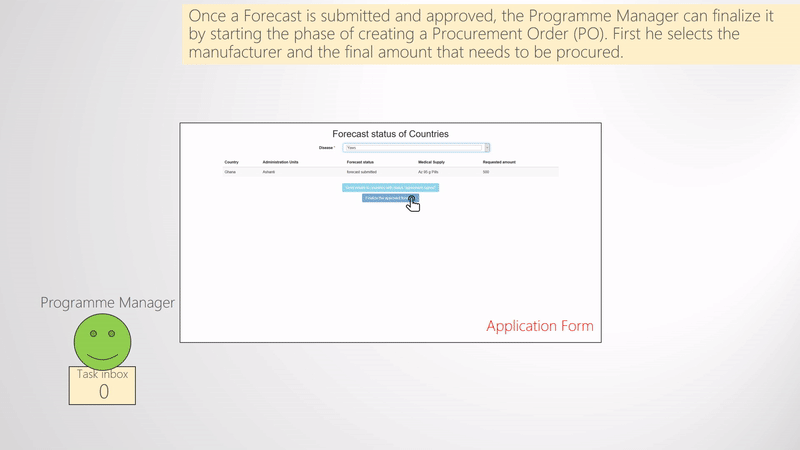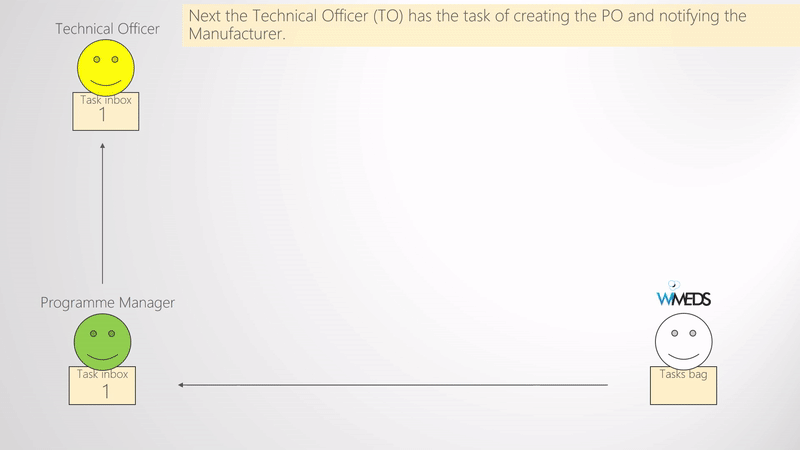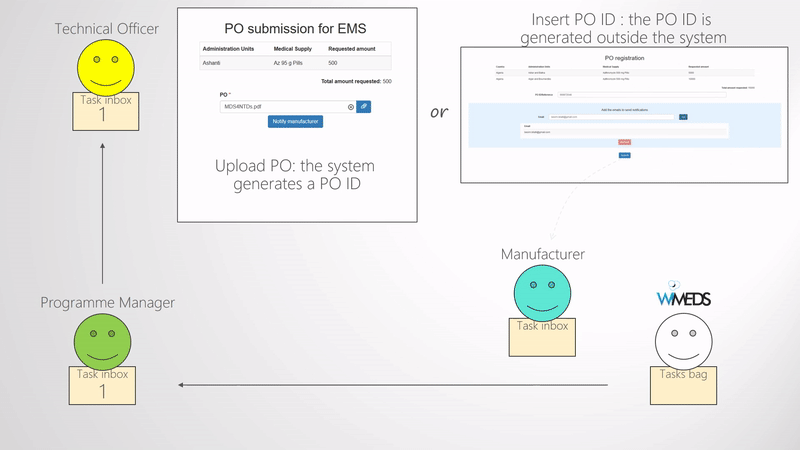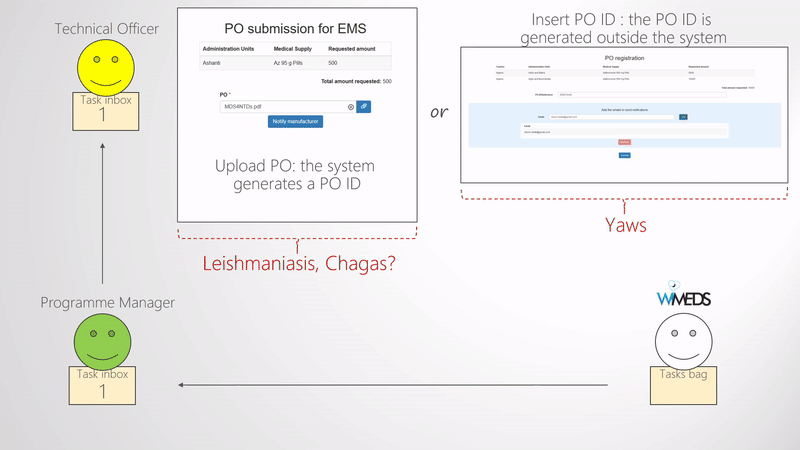
Finalize forecast and create procurement order
When enough forecasts are submitted and approved by the the NTD Programme Manager, they need to be finalized. The finalization of the forecast consists of selecting the Manufacturer for each Forecast and potentially deciding the final quantity of medical supplies to be procured (e.g., if some changes need to be applied). Once these are set and submitted, the process of the Procurement of the medical supplies starts. That is, new tasks are assigned by the NTD Programme Manager to the NTD Technical Officer, who is responsible of the procurement. Thus, the Technical Officer receives a task per country and manufacturer where he uploads the Procurement Order and notifies the manufacturer. A schematic representation of the above mentioned is shown in the Finalize forecast and create procurement order figure. As shown in the figure, the Technical Officer inserts the Procurement Order ID and uploads the corresponding document to WIMEDS. Note that the Procurement ID is important since different actors can transparently track the state of the Procurement through its ID.
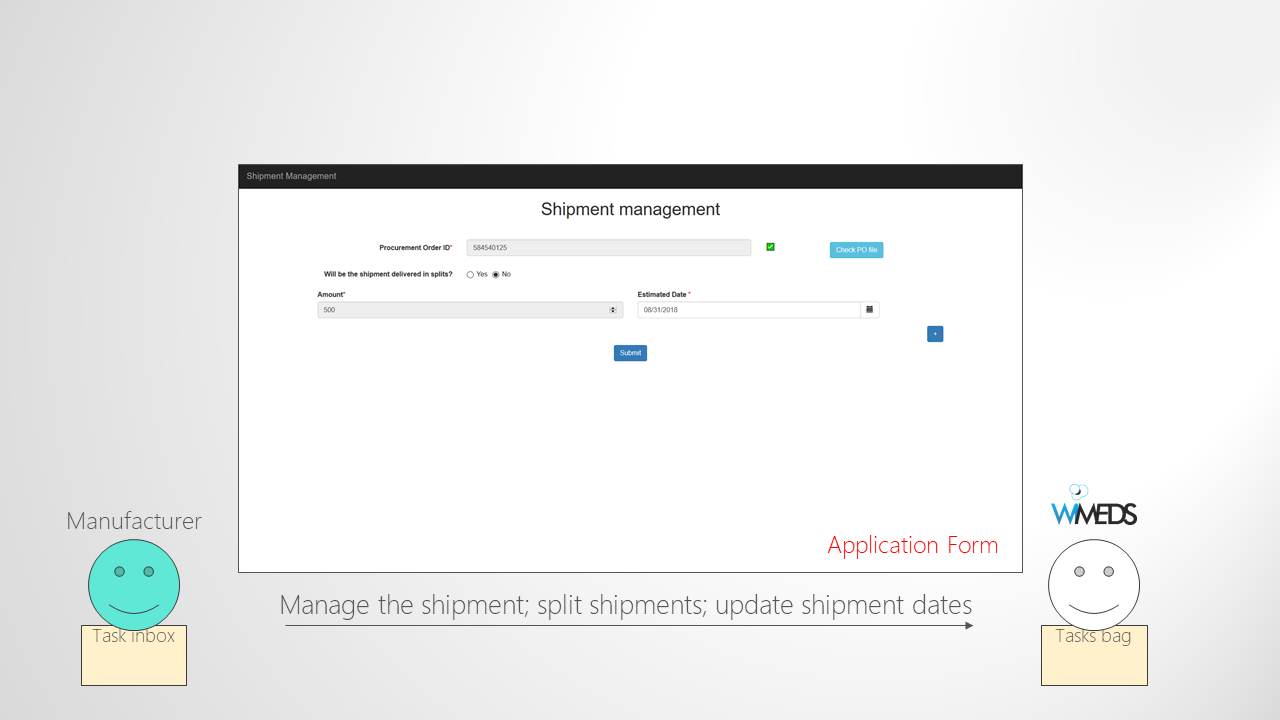
Create Shipment and Insert Delivery Dates
Finally, when procurement orders are created for different countries, the manufacturer is notified. Yet, since the manufacturer is not always able to reply all at once to the demands, it can access an application in order to confirm the available medical supplies. That is, it can provide the quantity of the medical supplies that can be served at the moment. When this quantity is submitted by the manufacturer, the NTD Programme Manager has the task of splitting this quantity among different procurement orders. Next, the manufacturer can see the splits assigned to each country and can immediately start the process of shipping the medical supplies to the corresponding country. However, before physically dispatching the medical supplies, the manufacturer needs to get a green light from the country. Thus the process is performed as follows. The manufacturer starts the process of shipping the medical supplies by uploading all the necessary documents required for getting the green light. The Country Office is notified by email and receives a task where he can check all the documents and if they are correct can give the green light. If the green light is given, the manufacturer gets a notification email and receives the task of providing the details about the shipment (e.g., estimated date of delivery, flight number, etc.). Finally, when this task is completed by the manufacturer, the Country Office receives the final task of confirming the reception of the medical supplies. Note that this task contains all the information to track the shipment, but once the medical supplies are received by the country, by completing this task, the Country Office informs all the actors that the medical supplies are already received in the country.